VisionArray ® Chip for MYCO Genotyping
The mycobacterial genera comprise more than 140 species, which, for the purpose of diagnosis and treatment, have been grouped into three categories: M. tuberculosis complex (MTC), M. leprae, and non-tuberculous mycobacteria (NTM). The majority of the Mycobacterium species belongs to the NTM group and can be found in different environments. Many of these bacteria cause life-threatening infections in humans and in recent years, the mortality and morbidity associated with NTMs has increased especially in immunocompromised patients worldwide. Treatment of NTMs is specific to each species and therefore a clear distinction between the present species is of extreme importance. Reliable and rapid molecular diagnostics are the basis of an adequate therapy that is given by the VisionArray® MYCO Chip 2.0.
VisionArray ® MYCO Chip 2.0
Chip Description
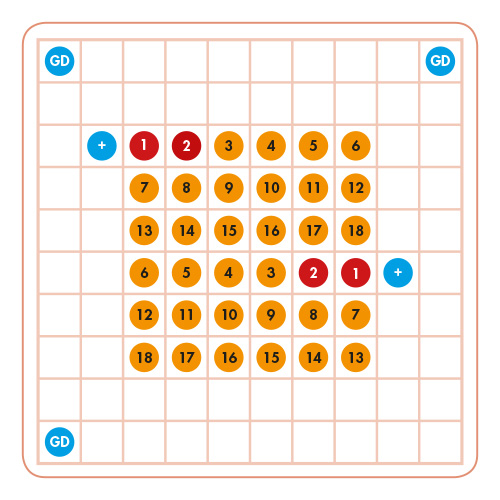
The VisionArray® MYCO Chip 2.0 is designed to detect several clinically relevant mycobacterial species. All capture sequences and the positive control are set up on the Chip as duplicates and the guide dots as triplicates. The signals are visible on the Chip as dark blue areas. The automated evaluation of the results is performed by a VisionArray® Software.
Ordering Information
|
Prod. No.:
|
Tests: |
Registration Status¹: |
|
VA-0005-10
|
10 |
 |
| 1 |
 In vitro diagnostic medical device according to EU directive 98/79/EC. In vitro diagnostic medical device according to EU directive 98/79/EC.
CE IVD only available in certain countries. All other countries research use only!
Please contact your local dealer for more information. |
Download Information
VisionArray ® MYCO PreCise Master Mix 2.0
The VisionArray ® MYCO PreCise Master Mix 2.0 is intended to be used to amplify and biotinylate specific sections of the ITS and, in case of the M. tuberculosis complex, IS6110 region as well as the SR4 region (Zozaya-Valdés et al. 2017) of mycobacterial genomes by polymerase chain reaction (PCR) using DNA samples extracted from e.g. clinical specimens, pulmonary smears or cultivated samples. The VisionArray ® MYCO PreCise Master Mix 2.0 is designed to amplify mycobacteria including but not limited to those detected by the corresponding VisionArray ® MYCO Chips and, if present in the DNA sample, genomic sequences of the human HLA-DQA1 gene as a PCR positive control.
Ordering Information
|
Prod. No.:
|
Tests: |
Registration Status¹: |
|
ES-0008-50
|
50 |
 |
| 1 |
 In vitro diagnostic medical device according to EU directive 98/79/EC. In vitro diagnostic medical device according to EU directive 98/79/EC.
CE IVD only available in certain countries. All other countries research use only!
Please contact your local dealer for more information. |
Download Information
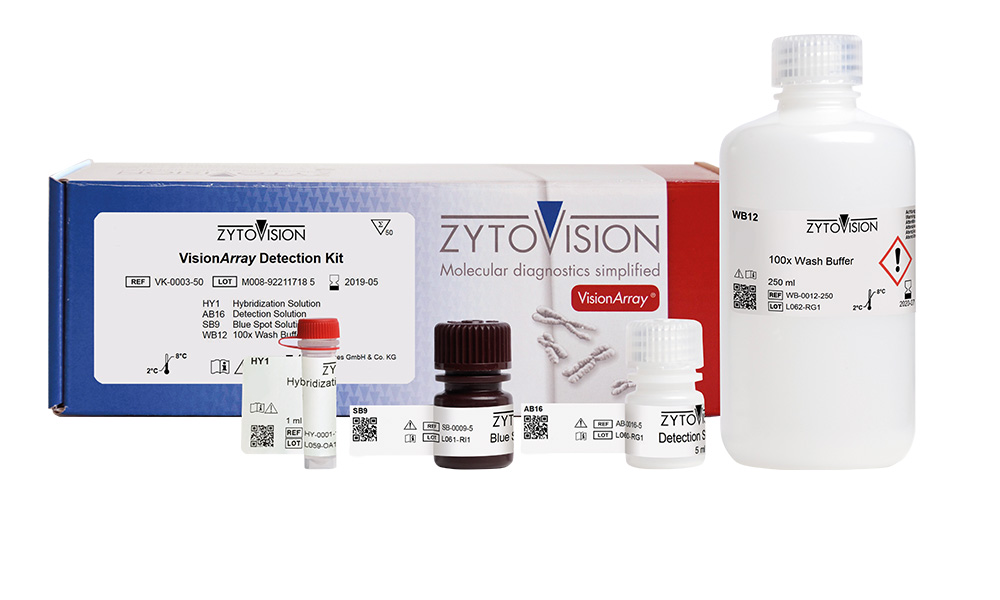
VisionArray ® Detection Kit
The VisionArray® Detection Kit is intended to be used with a VisionArray® PreCise Master Mix and the corresponding VisionArray® DNA Chip for the qualitative detection of specific DNA sequences. The automated analysis has to be performed with a VisionArray® Software. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
Ordering Information
|
Prod. No.:
|
Tests: |
Registration Status¹: |
|
VK-0003-50
|
50 |
 |
| 1 |
 In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746.
CE IVD only available in certain countries. All other countries research use only!
Please contact your local dealer for more information. |
Download Information
VisionArray ® Software
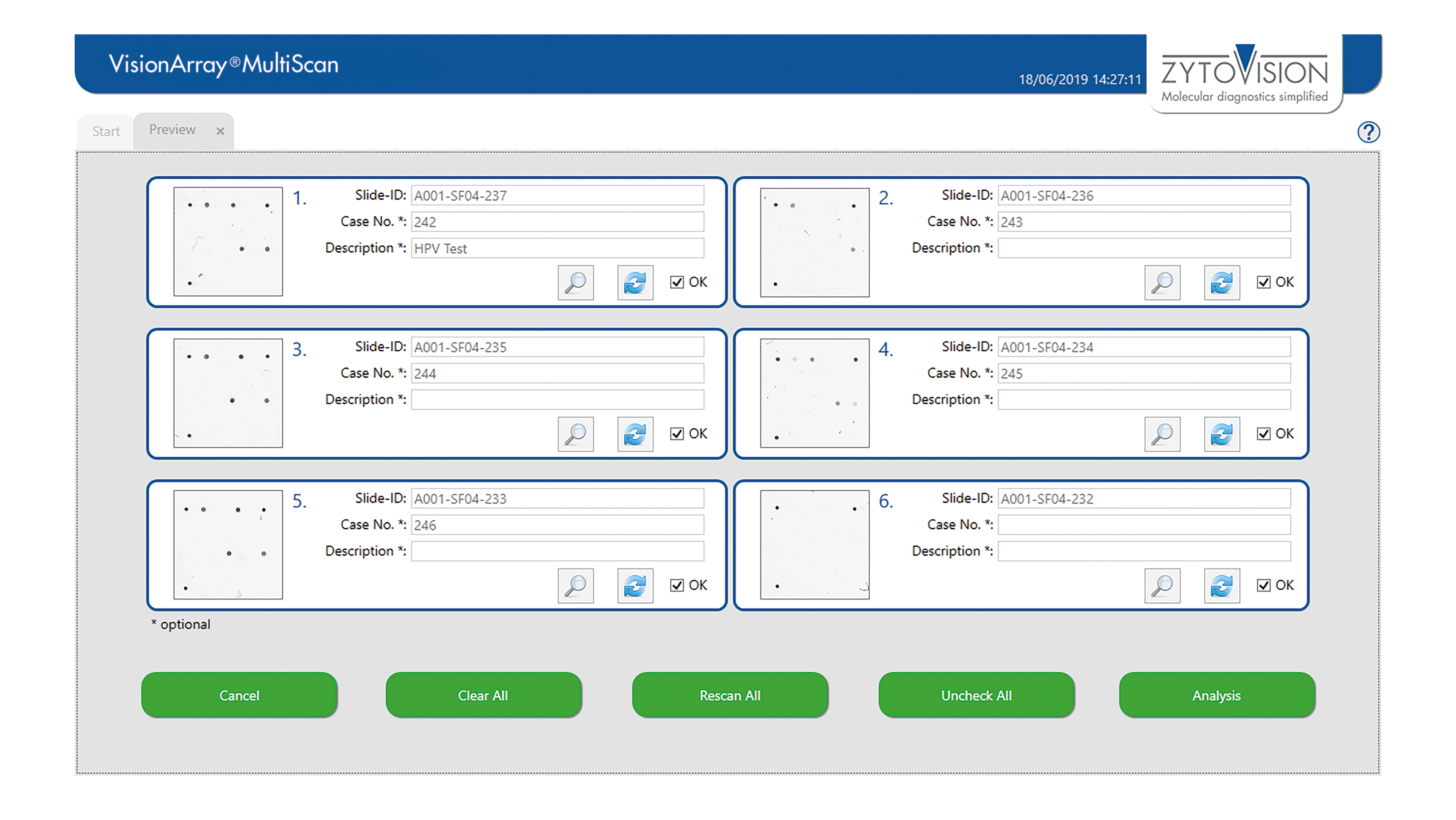
VisionArray ® MultiScan Software
The VisionArray® MultiScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.:
|
Registration Status¹: |
|
E-4302-1
|
 |
| 1 |
 In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746.
CE IVD only available in certain countries. All other countries research use only!
Please contact your local dealer for more information. |
Download Information
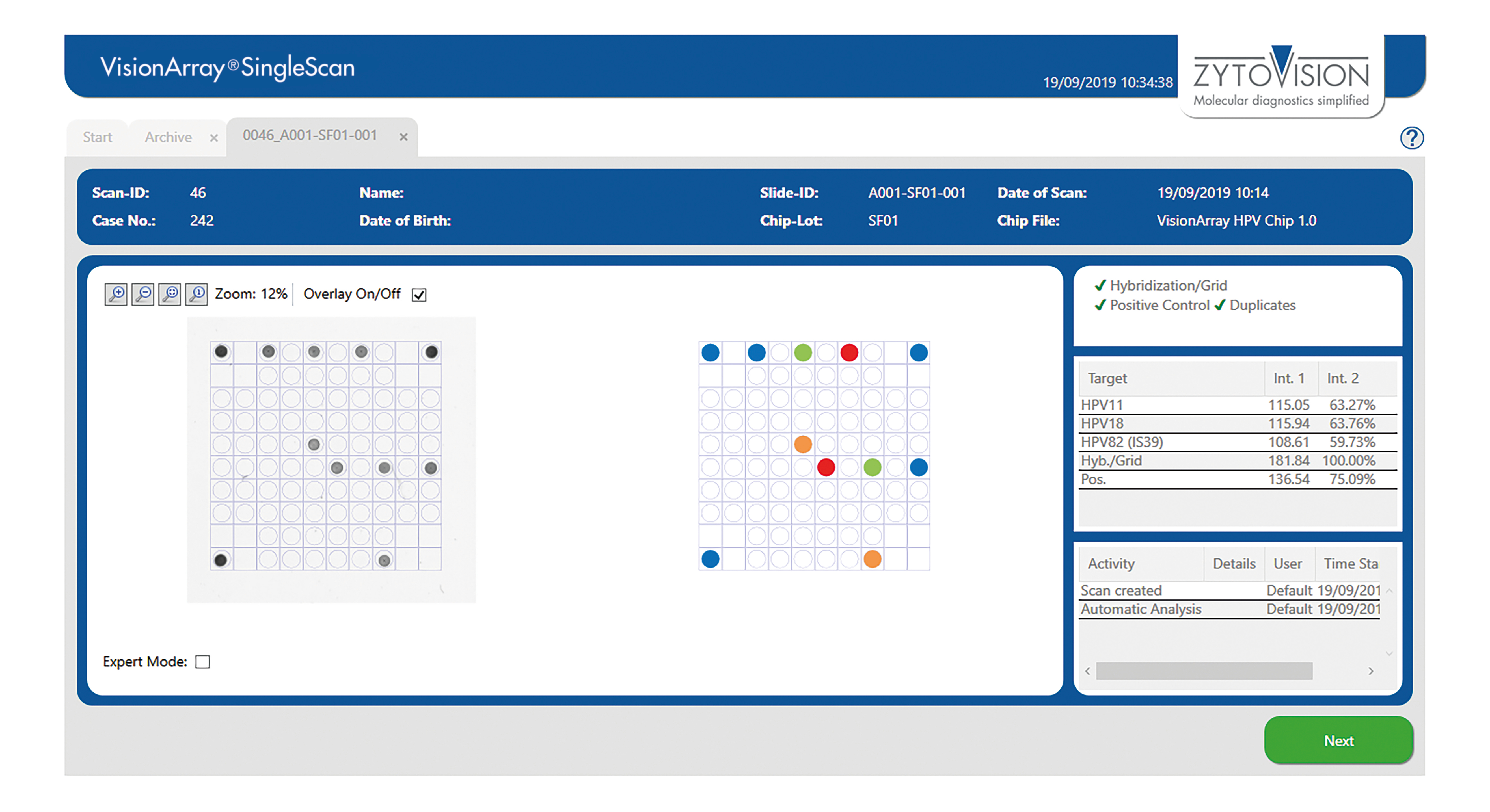
VisionArray ® SingleScan Software
The VisionArray® SingleScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.:
|
Registration Status¹: |
|
E-4301-1
|
 |
| 1 |
 In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746.
CE IVD only available in certain countries. All other countries research use only!
Please contact your local dealer for more information. |
Download Information

 In vitro diagnostic medical device according to EU directive 98/79/EC.
In vitro diagnostic medical device according to EU directive 98/79/EC. 

 PRODUCTS
PRODUCTS















 In vitro diagnostic medical device according to IVDR (EU) 2017/746.
In vitro diagnostic medical device according to IVDR (EU) 2017/746.