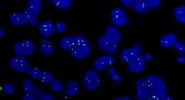
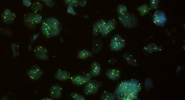
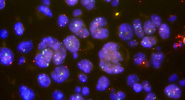
Image Gallery
Click to enlarge
| 1 |  In vitro diagnostic medical device according to EU directive 98/79/EC. In vitro diagnostic medical device according to EU directive 98/79/EC. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |


 PRODUCTS
PRODUCTS